209860-87-7
- Product Name:Tafluprost
- Molecular Formula:C25H34F2O5
- Purity:99%
- Molecular Weight:452.539
Product Details:
CasNo: 209860-87-7
Molecular Formula: C25H34F2O5
Buy High Purity 99% High Grade Tafluprost 209860-87-7 Safe Delivery
- Molecular Formula:C25H34F2O5
- Molecular Weight:452.539
- Vapor Pressure:4.62E-13mmHg at 25°C
- Refractive Index:1.548
- Boiling Point:552.9ºC at 760mmHg
- PKA:14.48±0.70(Predicted)
- Flash Point:288.2°C
- PSA:75.99000
- Density:1.186 g/cm3
- LogP:4.68300
Tafluprost(Cas 209860-87-7) Usage
| Category | Prostaglandin Analog, Ophthalmic Medication |
| Definition | Tafluprost is a synthetic prostaglandin analogue primarily used in ophthalmology to reduce intraocular pressure (IOP) in patients with open-angle glaucoma and ocular hypertension. It works by acting as an agonist at prostaglandin F receptors, leading to increased aqueous humor outflow and, consequently, lower intraocular pressure. |
|
Uses |
Hangzhou Huarong Pharm Co., Ltd. established since 2009 , has been always focusing on supplying products and services to our clients in the field of small molecule drug. Antibody-drug Conjugates (ADCs) and others. To maintain a high innovation efficiency, the company has continuously increased the investment on R&D facilities and state-of-the-art equipment in the past several years, including the establishment of kilogram GMP conditions plants and R&D centers. Tafluprost is used to treat open-angle glaucoma, the most common type of glaucoma, which occurs when the eye’s drainage canals become clogged, increasing IOP. It is also used to manage elevated intraocular pressure, a condition where the pressure in the eye is higher than normal, increasing the risk of glaucoma and vision loss. |
| Pharmacokinetics | Tafluprost is rapidly absorbed through the cornea, where it undergoes hydrolysis to the active form. About 75% of tafluprost is absorbed by the cornea after topical administration. It is metabolized by beta-oxidation and further oxidation, with a unique resistance to degradation due to its fluorine atoms. |
| Mechanism of Action | Once tafluprost penetrates the cornea, it is hydrolyzed by esterases to its active acid form, tafluprost acid, which has a high affinity for prostaglandin F receptors. By stimulating these receptors, it increases the outflow of aqueous humor from the eye, reducing IOP. |
|
Brand name |
Zioptan® (FDA Approved in 2012) Taflotan® (International Brand) |
| FDA Approval | Tafluprost, marketed under the brand name Zioptan, was approved by the FDA on February 10, 2012, for the treatment of glaucoma and ocular hypertension. It is available as an ophthalmic solution. |
InChI:InChI=1/C25H34F2O5/c1-18(2)32-24(30)13-9-4-3-8-12-20-21(23(29)16-22(20)28)14-15-25(26,27)17-31-19-10-6-5-7-11-19/h3,5-8,10-11,14-15,18,20-23,28-29H,4,9,12-13,16-17H2,1-2H3/b8-3+,15-14+
209860-87-7 Relevant articles
Tafluprost once daily for treatment of elevated intraocular pressure in patients with open-angle glaucoma
Yang Liu &Weiming Mao
, Clinical Ophthalmology Volume 7, 2013 - Issue
Nakajima et al first reported the intraocular pressure-lowering effects of tafluprost in naïve experimental cynomolgus monkeys.Citation6 In that study one eye was treated with 20 μL of 0.0005% tafluprost or 0.005% latanoprost, while the fellow eye was treated with vehicle control. The authors found that treatment with tafluprost resulted in a significant intraocular pressure reduction of up to about 2 mmHg 4–8 hours following treatment, which was equivalent to the effect of latanoprost.
A Comparative, Placebo-Controlled Study of Prostanoid Fluoroprostaglandin-Receptor Agonists Tafluprost and Latanoprost in Healthy Males
Andrew Sutton, Anne Gilvarry, and Auli Ropo
Paragraph 0101-0103, (2021/05/29)
This was a phase I study in healthy males 18–45 years of age (N = 49). Participants were randomized to receive 1 of 4 eye drops: tafluprost 0.0025% or 0.005%, latanoprost 0.005%, or a placebo, administered once-daily for 7 days, with 1 drop per eye.
Tafluprost: The First Preservative-Free Prostaglandin to Treat Open-Angle Glaucoma and Ocular Hypertension
Cory Swymer and Michael W Neville
, Annals of pharmacotherapy, Volume 46, Issue 11,2012
Tafluprost 0.0015% is the first topical prostaglandin approved by the Food and Drug Administration for treatment of open-angle glaucoma and ocular hypertension that does not contain the widely used preservative, benzalkonium chloride (BAK). Although some controversy surrounds the long-term safety of exposure to BAK, clinical trial data are inconclusive.
209860-87-7 Process route
-
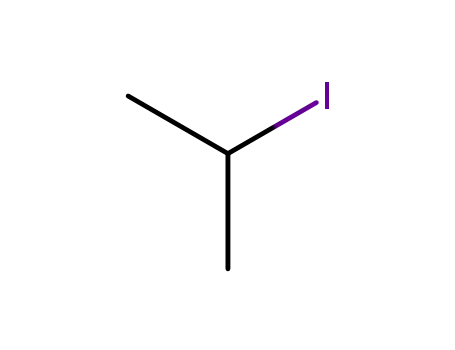
- 75-30-9
2-iodo-propane

-
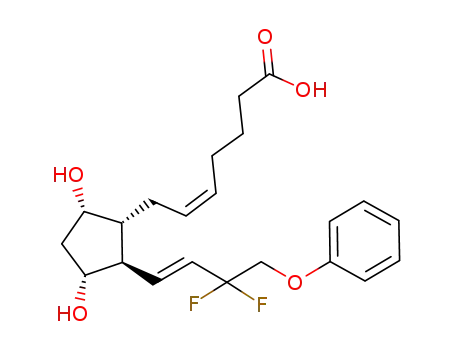
- 209860-88-8
AFP-172

-
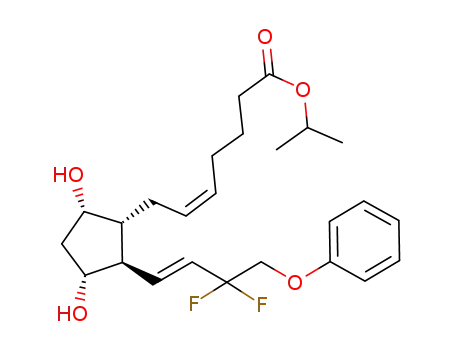
- 209860-87-7
tafluprost
| Conditions | Yield |
|---|---|
|
AFP-172; With 1,8-diazabicyclo[5.4.0]undec-7-ene; In acetone; at 0 ℃; Inert atmosphere;
2-iodo-propane; at 0 - 20 ℃; for 21h; Inert atmosphere;
|
83% |
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In acetone; at 5 - 30 ℃; Inert atmosphere;
|
82% |
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In acetone; at 20 ℃; for 16h;
|
|
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In acetone; at 20 ℃; for 16h;
|
|
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In acetone; at 20 ℃;
|
|
|
With potassium carbonate; In N,N-dimethyl-formamide; at 80 ℃; for 2h; Inert atmosphere;
|
6.8 g |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 80 ℃; for 2h; Inert atmosphere;
|
6.8 g |
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In acetone; at 20 ℃; for 22h;
|
9 mg |
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In acetone; at 28 - 32 ℃; for 5h;
|
29.85 g |
-
![(Z)-7-{(1R,2R,3R,5S)-3-[(tert-butyldimethylsilanyl)oxy]-2-((E)-3,3-difluoro-4-phenoxybut-1-en-1-yl)-5-hydroxy-cyclopentyl} hepta-5-enoic acid isopropyl ester](/upload/2024/1/bb35e769-c186-4aec-b47e-c693a5b7d684.png)
-
(Z)-7-{(1R,2R,3R,5S)-3-[(tert-butyldimethylsilanyl)oxy]-2-((E)-3,3-difluoro-4-phenoxybut-1-en-1-yl)-5-hydroxy-cyclopentyl} hepta-5-enoic acid isopropyl ester

-

- 209860-87-7
tafluprost
| Conditions | Yield |
|---|---|
|
With tetrabutyl ammonium fluoride; In tetrahydrofuran; at 0 - 20 ℃; for 3.5h;
|
90% |
|
With tetrabutyl ammonium fluoride; In tetrahydrofuran; at 0 - 20 ℃; for 3.5h; Inert atmosphere;
|
90% |
209860-87-7 Upstream products
-
75-30-9

2-iodo-propane
-
209860-88-8

AFP-172
-
40665-68-7

dimethyl (3-phenoxy-2-oxopropyl)phosphonate
-
39746-01-5

(1S,5R,6R,7R)-6-formyl-7-benzoyloxy-2-oxabicyclo[3.3.0]octan-3-one
Relevant Products
-
Pralsetinib
CAS:2097132-94-8
-
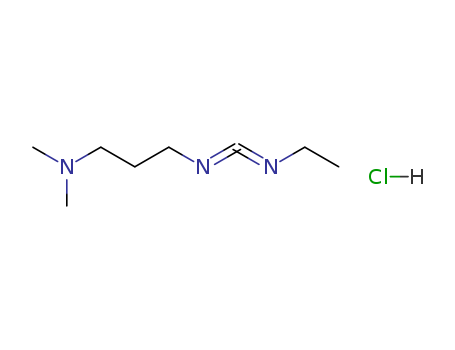
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
CAS:25952-53-8
-
Ondansetron Hcl
CAS:103639-04-9