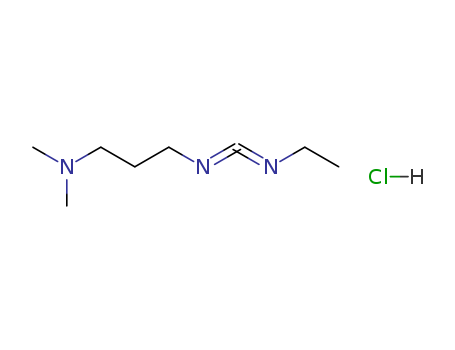
25952-53-8
- Product Name:1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
- Molecular Formula:C8H18ClN3
- Purity:99%
- Molecular Weight:191.704
Product Details:
CasNo: 25952-53-8
Molecular Formula: C8H18ClN3
Appearance: White crystalline powder
To see more products, please check our platform website--www.huarongpharm.com
Reliable Quality 25952-53-8 Efficient Transportation, Factory Supply High Purity 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
- Molecular Formula:C8H18ClN3
- Molecular Weight:191.704
- Appearance/Colour:White crystalline powder
- Vapor Pressure:0.171mmHg at 25°C
- Melting Point:110-115 °C(lit.)
- Refractive Index:n20/D 1.461
- Boiling Point:197.7 °C at 760 mmHg
- Flash Point:73.4 °C
- PSA:27.96000
- Density:0.877g/mLat 20°C(lit.)
- LogP:1.93390
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride(Cas 25952-53-8) Usage
|
Description |
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride is a water-soluble carbodiimide used to couple carboxyl groups to primary amines without introducing a spacer molecule, making it an essential tool in biochemical and organic reactions. It is particularly important in peptide synthesis, esterification, and crosslinking reactions. It is also used as a corrosion inhibitor for mild steel in acidic environments. |
|
Uses |
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (commonly referred to as EDC, EDAC, or EDCI) is classified as a carbodiimide reagent and a zero-length crosslinking agent used primarily in organic synthesis and biochemistry. It is also categorized as a corrosion inhibitor in certain applications. EDC is primarily used as a coupling reagent in the synthesis of peptides by facilitating the formation of amide bonds between carboxyl groups and primary amines. It is used in the preparation of immunoconjugates for antibody and hapten-carrier protein conjugation. |
| Corrosion Inhibition | 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride has been investigated as a corrosion inhibitor for mild steel, using techniques like weight loss measurement, electrochemical impedance spectroscopy (EIS), and polarization studies. The compound adsorbs onto the steel surface, acting as a mixed-type inhibitor that reduces both anodic and cathodic reactions, with high inhibition efficiency at low concentrations. |
|
Synthesis |
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) can be synthesized by reacting 3-dimethylaminopropylamine with ethyl isocyanate, followed by the formation of the hydrochloride salt. It is commonly used in catalytic amounts to synthesize esters and amides from carboxylic acids, often in the presence of catalysts like dimethylaminopyridine (DMAP). |
InChI:InChI=1/C7H17N3.ClH/c1-4-8-9-6-5-7-10(2)3;/h4-7H2,1-3H3;1H/b9-8+;
25952-53-8 Relevant articles
Development of Sequential Injection Analysis for Determination of 1-(3-Dimethylaminopropyl)-3-Ethylcarbodiimide Hydrochloride (EDC・HCl) Based on Condensation Reaction between Malonic Acid and Ethylenediamine
Kunihiko Seno, Kazuki Matsumura, Koji Oshita, Mitsuko Oshima, Shoji Motomizu
Paragraph 0025; 0034; 0035; 0039; 0040; 0044, (2009/03/08)
A sequential injection analysis (SIA) using a specific condensation reaction between malonic acid and ethylenediamine with 1–(3–dimethylaminopropyl)–3–ethylcarbodiimide hydrochloride (EDC・HCl) in aqueous media was developed for EDC・HCl determination. EDC・HCl can act as a dehydration or condensation reagent for the formation of amide (peptide). The automated SIA for EDC・HCl determination has improve the reaction efficiency and the sensitivity by coupling with forward and backward mixing in the reaction coil.
Spectrophotometric Determination of 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride by Flow Injection Analysis
Kunihiko SENO, Kazuki MATUMURA, Mitsuko OSHIMA, Shoji MOTOMIZU
Analytical Sciences, 2008
The absorbances were measured at 400 nm and the reaction was accelerated at 40°C. The calibration graph showed good linearity from 0 to 10% of EDC·HCl solutions: the regression equation was y = 3.15 × 104x (y, peak area; x, % concentration of EDC·HCl). The RSD was under 1.0%. Sample throughput was 15 h-1.
An exhaustive study of a coupling reagent (1-(3-dimethylaminopropyl) 3-ethylcarbodiimide hydrochloride) as corrosion inhibitor for steel
Dahiya, Shefali Kumar, Parmod Lata, Suman Kumar, Raman Dahiya, Naveen Ahlawat, Suman
, Indian Journal of Chemical Technology (IJCT) IJCT Vol.24 [2017]
The corrosion inhibition of mild steel in 0.5 M HCl by a coupling reagent, 1-(3-Dimethylaminopropyl) 3-ethylcarbodiimide hydrochloride ( EDC), has been investigated by weight loss, electrochemical impedance spectroscopy (EIS), polarization technique, theoretical studies of quantum chemistry and scanning electron microscopy (SEM). Experimental results show that the reagent is an effective inhibitor for the corrosion of mild steel in acidic solution and it exhibited > 90% inhibition efficiency at low concentrations of the inhibitor. Potentiostatic polarization studies show that the studied compound is a mixed-type inhibitor. The results of EIS show that EDC inhibits corrosion of mild steel by adsorption mechanism. Surface morphology has been studied by SEM and the theoretical quantum calculations also are in agreement with the experimental results. The adsorption of inhibitor on the mild steel surface follow Langmuir adsorption isotherm. The thermodynamic parameters of corrosion process have also been calculated and discussed
25952-53-8 Upstream products
-
628-13-7

pyridine hydrochloride
-
1892-57-5

N-(3-dimethylaminopropyl)-N-ethylcarbodiimide
-
18997-72-3

N-(3-Dimethylamino-propyl)-dithiocarbamidsaeure
-
27421-70-1

3-(dimethylamino)propyl isothiocyanate
25952-53-8 Downstream products
-
81409-90-7

cabergoline
-
81409-91-8

N-<<<3-(dimethylamino)propyl>amino>carbonyl>-N-ethyl-6-(2-propenyl)-ergoline-8β-carboxamide
-
289903-64-6

homovanillic acid pentafluorophenyl ester
-
454452-97-2

5-fluoro-2-methyl-3-(N-(S-α-hydroxylmethyl)benzyl)-indenylacetamide
Relevant Products
-
Milvexian
CAS:1802425-99-5
-
Terbinafine Hcl
CAS:78628-80-5
-
Tafluprost
CAS:209860-87-7








