
1297538-32-9
- Product Name:Darolutamide
- Molecular Formula:C19H19ClN6O2
- Purity:99%
- Molecular Weight:398.852
Product Details:
CasNo: 1297538-32-9
Molecular Formula: C19H19ClN6O2
Buy Quality Darolutamide,Hot Sale 1297538-32-9 Safe Transportation
- Molecular Formula:C19H19ClN6O2
- Molecular Weight:398.852
- Boiling Point:719.5±60.0 °C(Predicted)
- PKA:11.10±0.10(Predicted)
- PSA:119.62000
- Density:1.41±0.1 g/cm3(Predicted)
- LogP:3.06098
Darolutamide (Cas 1297538-32-9) Usage
Darolutamide, marketed as Nubeqa, is a unique androgen receptor antagonist developed for the treatment of prostate cancer. As an antiandrogen medication, it is specifically used in the treatment of non-metastatic castration-resistant prostate cancer in men. Darolutamide has received approval for addressing this condition in conjunction with surgical or medical castration.
Belonging to the class of antiandrogens, Darolutamide is distinct as a structurally unique androgen receptor antagonist. It operates as a type of chemotherapy and antineoplastic (anticancer) drug. The brand name Nubeqa is associated with Darolutamide, which plays a crucial role in managing non-metastatic castration-resistant prostate cancer, providing an important therapeutic option for individuals with this specific form of prostate cancer.
Hangzhou Huarong Pharm Co., Ltd. established since 2009 , has been always focusing on supplying products and services to our clients in the field of small molecule drug. Huarong Pharm has built platforms for the research, development and manufacturing of Building Blocks, Reference Compounds & Impurities, Natural products, APIs & Intermediates, Antibody-drug Conjugates (ADCs) and others.
1297538-32-9 Relevant articles
Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer
Karim Fizazi, M.D., Neal Shore, M.D., Teuvo L. Tammela, M.D., Ph.D., Albertas Ulys, M.D., Egils Vjaters, M.D., Sergey Polyakov, M.D., Mindaugas Jievaltas, M.D
, N Engl J Med 2019;380:1235-1246
In total, 1509 patients underwent randomization (955 to the darolutamide group and 554 to the placebo group). Among men with nonmetastatic, castration-resistant prostate cancer, metastasis-free survival was significantly longer with darolutamide than with placebo. The incidence of adverse events was similar for darolutamide and placebo.
Clinical Development of Darolutamide: A Novel Androgen Receptor Antagonist for the Treatment of Prostate Cancer
Karim Fizazi 1 , Matthew R. Smith 2 , Bertrand Tombal 3
, Clinical Genitourinary Cancer Volume 16, Issue 5 , October 2018, Pages 332-340
A Multinational, Randomized, Double-Blind, Placebo-Controlled, Phase III Efficacy and Safety Study of Darolutamide [ODM-201] in Men With High-Risk Non-metastatic Castration-Resistant Prostate Cancer) study, metastasis-free survival is being evaluated in men with nonmetastatic CRPC who will receive ADT in combination with darolutamide or placebo.
Overall survival and adverse events after treatment with darolutamide vs. apalutamide vs. enzalutamide for high-risk non-metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis
Mike Wenzel, Luigi Nocera, Claudia Collà Ruvolo, Christoph Würnschimmel, Zhe Tian, Shahrokh F. Shariat, Fred Saad, Derya Tilki, Markus Graefen, Luis A. Kluth, Alberto Briganti, Philipp Mandel, Francesco Montorsi, Felix K. H. Chun & Pierre I. Karakiewicz
, Prostate Cancer and Prostatic Diseases volume 25, pages139–148 (2022)
We performed a systematic review and network meta-analysis focusing on OS and AE according to the most recent apalutamide, enzalutamide, and darolutamide reports. We systematically examined and compared apalutamide vs. enzalutamide vs. darolutamide efficacy and toxicity, relative to ADT according to PRISMA. We relied on PubMed search for most recent reports addressing prospective randomized trials with proven predefined OS benefit, relative to ADT: SPARTAN, PROSPER, and ARAMIS. OS represented the primary outcome and AEs represented secondary outcomes.
1297538-32-9 Process route
-
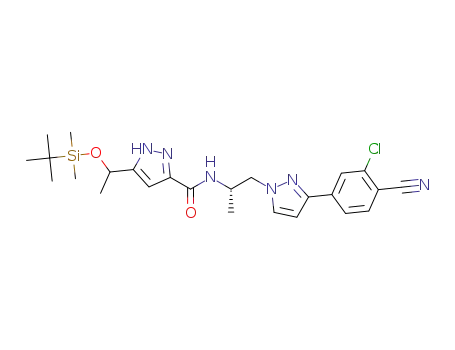
-
5-(1-((tert-butyldimethylsilyl)oxy)ethyl)-N-((S)-1-(3-(3-chloro-4-cyanophenyl)-1H-pyrazol-1-yl)propan-2-yl)-1H-pyrazole-3-carboxamide

-
![N-{(2S)-1-[3-(3-chloro-4-cyanophenyl)-1H-pyrazol-1-yl]propan-2-yl}-5-[(1RS)-1-hydroxyethyl]-1H-pyrazole-3-carboxamide](/upload/2024/1/83dcc71e-7b86-4503-94ab-19e5b5295b10.png)
- 1297538-32-9
N-{(2S)-1-[3-(3-chloro-4-cyanophenyl)-1H-pyrazol-1-yl]propan-2-yl}-5-[(1RS)-1-hydroxyethyl]-1H-pyrazole-3-carboxamide
| Conditions | Yield |
|---|---|
|
With tetrabutyl ammonium fluoride; In tetrahydrofuran; at 0 ℃; for 6h;
|
92.9% |
-
-
C21H21ClN6O3

-
![N-{(2S)-1-[3-(3-chloro-4-cyanophenyl)-1H-pyrazol-1-yl]propan-2-yl}-5-[(1RS)-1-hydroxyethyl]-1H-pyrazole-3-carboxamide](/upload/2024/1/83dcc71e-7b86-4503-94ab-19e5b5295b10.png)
- 1297538-32-9
N-{(2S)-1-[3-(3-chloro-4-cyanophenyl)-1H-pyrazol-1-yl]propan-2-yl}-5-[(1RS)-1-hydroxyethyl]-1H-pyrazole-3-carboxamide
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In ethanol; water; at 20 ℃;
|
93.3% |
1297538-32-9 Upstream products
-
154607-01-9
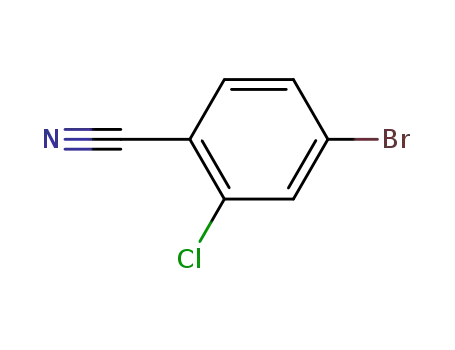
4-bromo-2-chlorobenzonitrile
-
1297537-35-9
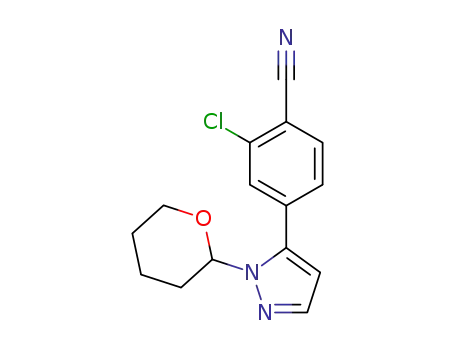
2-Chloro-4-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-5-yl)benzonitrile
-
1297537-39-3
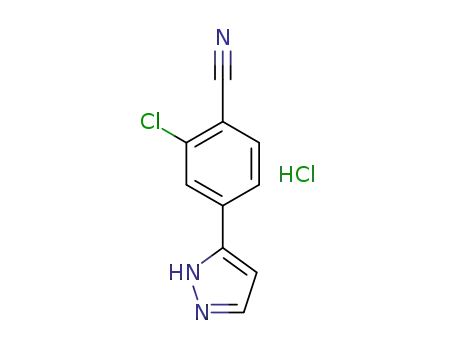
2-Chloro-4-(1H-pyrazol-5-yl)benzonitrile hydrochloride
-
1297537-37-1
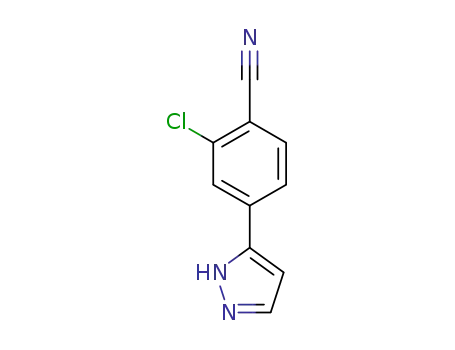
2-chloro-4-(1H-pyrazol-5-yl)benzonitrile
1297538-32-9 Downstream products
-
1297540-05-6
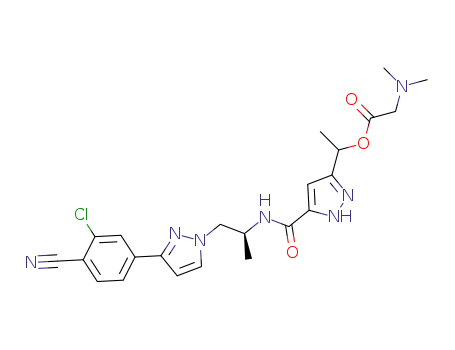
1-(5-((S)-1-(3-(3-chloro-4-cyanophenyl)-1H-pyrazol-1-yl)propan-2-ylcarbamoyl)-1H-pyrazol-3-yl)ethyl 2-(dimethylamino)acetate
-
1403334-18-8
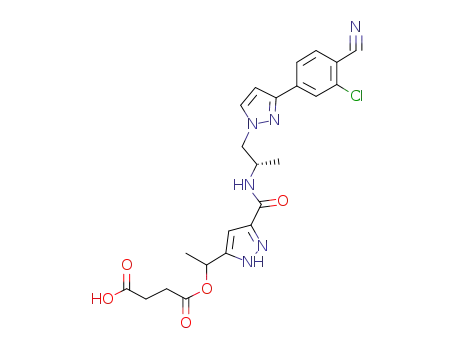
4-(1-(3-((S)-1-(3-(3-Chloro-4-cyanophenyl)-1H-pyrazol-1-yl)propan-2-yl-carbamoyl)-1H-pyrazol-5-yl)ethoxy)-4-oxobutanoic acid
Relevant Products
-
Milvexian
CAS:1802425-99-5
-
Brivaracetam
CAS:357336-20-0








