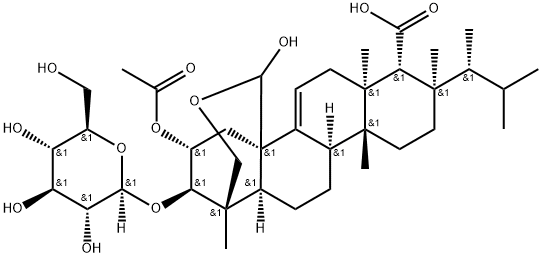
260979-95-1
- Product Name:Enfumafungin
- Molecular Formula:C38H60O12
- Purity:99%
- Molecular Weight:708.89
Product Details:
CasNo: 260979-95-1
Molecular Formula: C38H60O12
Appearance: White to off-white solid
Delivery Time: 2 weeks after order
Throughput: 100KG/Month
Purity: 99%
Synonyms: 4H-1,4a-Propano-2H-phenanthro[1,2-c]pyran-7-carboxylic acid, 14-(acetyloxy)-8-[(1R)-1,2-dimethylpropyl]-15-(β-D-glucopyranosyloxy)-1,6,6a,7,8,9,10,10a,10b,11,12,12a-dodecahydro-4-hydroxy-1,6a,8,10a-tetramethyl-, (1S,4aR,6aS,7R,8R,10aR,10bR,12aS,14R,15R)-; (1S,2R,3R,4aR,6aS,7R,8R,10aR,10bR,12aS)-3-acetoxy-13-hydroxy-1,6a,8,10a-tetramethyl-8-((R)-3-methylbutan-2-yl)-2-(((2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-2,3,4,6,6a,7,8,9,10,10a,10b,11,12,12a-tetradecahydro-1H-1,4a-(methanooxymethano)chrysene-7-carboxylic acid; (1S,2R,3R,4aR,6aS,7R,8R,10aR,10bR,12aS)-3-Acetoxy-13-hydroxy-1,6a,8,10a-tetramethyl-8-((R)-3-methylbutan-2-yl)-2-(((2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-1,3,4,6,6a,7,8,9,10,10a,10b,11,12,12a-tetradecahydro-2H-1,4a-(methanooxymethano)chrysene-7-carboxylic acid
Boiling point: 813.5±65.0 °C
Density: 1.31±0.1 g/cm3
Pka: 4.67±0.70
Definition:
Enfumafungin is a triterpene glycoside and hemiacetal isolated from a fermentation of Hormonema sp. and which specifically inhibits glucan synthesis in fungal cells. It has a role as an antifungal agent. It is a triterpenoid saponin, a monosaccharide derivative and a lactol.
Biological Activity:
Enfumafungin, a triterpene glycoside, was isolated from extracts of the species Hormonema. It is an antifungal compound that acts on the fungal cell wall as a (1,3)-beta-D-glucan synthase inhibitor.
Relevant Products
-
Decanoyl/octanoyl-glycerides
CAS:65381-09-1
-
Pneumocandin B0
CAS:135575-42-7
-
Alvimopan
CAS:156053-89-3