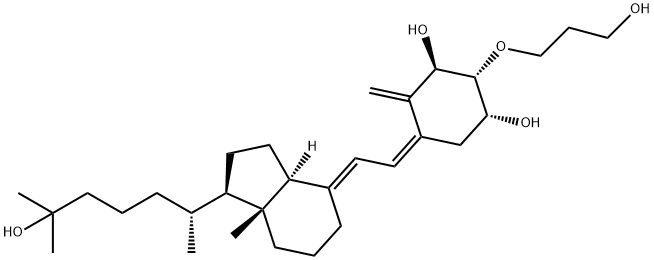
104121-92-8
- Product Name:Eldecalcitol
- Molecular Formula:C30H50O5
- Purity:99%
- Molecular Weight:490.71
Product Details:
CasNo: 104121-92-8
Molecular Formula: C30H50O5
Appearance: White to off-white powder
Delivery Time: 2 weeks after order
Throughput: 100KG/Month
Purity: 99%
Synonyms: 2-(3-hydroxypropoxy)-1,25-dihydroxyvitamin D3; ED-71; (1R,Z)-5-((E)-2-((1R,7aR)-1-((R)-6-hydroxy-6-methylheptan-2-yl)-7a-methylhexahydro-1H-inden-4(2H)-ylidene)ethylidene)-2-(3-hydroxypropoxy)-4-methylenecyclohexane-1,3-diol; 1alpha,25-dihydroxy-2beta-(3-hydroxypropoxy)vitamin D3; Eldecalcitol(ED-71); 2.beta.-(3-Hydroxypropoxy)-1.alpha.,25-dihydroxyvitaMin D3; (1S,2S,3S,5Z)-5-[(2E)-2-[(1R,3aS,7aR)-1-[(2R)-6-hydroxy-6-methylheptan-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-2-(3-hydroxypropoxy)-4-methylidenecyclohexane-1,3-diol
Boiling point: 655.7±55.0 °C
Density: 1.10 g/cm3
Vapor pressure: 0.0±4.5 mmHg at 25°C
Pka: 13.80±0.60
Solubility: Soluble in DMSO
Flash point: 350.3±31.5 °C
Refractive index: 1.550
Description:
Eldecalcitol (Edirol) was approved in January 2011 by the Japanese Ministry of Health, Labor, and Welfare for the treatment of osteoporosis. Because of vitamin D's central role in the bone health, vitamin D and analogs of vitamin D have been used to treat patients diagnosed with osteoporosis. Eldecalcitol is an analog of the active form of vitamin D, calcitriol, in which the lower cyclohexane ring contains a hydroxypropyl group. The synthesis of eldecalcitol involves the assembly of two units, a fully protected (3S,4S,5R)-oct-1-en-7-yne-3,4,5-triol and a fused bicyclic system, (R)-6- ((1R,3aR,7aR,E)-4-(bromomethylene)-7a-methyloctahydro-1H-inden-1- yl)-2-methylheptan-2-ol, through a Diels-Alder reaction to give fully protected eldecalcitol. The hydroxyl groups are then deprotected to give the parent molecule. Eldecalcitol binds to the vitaminDreceptor 2.7-fold more potently than calcitriol, while only weakly inhibiting serum parathyroid hormone.
Uses:
Eldecalcitol is a derivative of vitamin D3 (V676045) which is the vitamin that mediates intestinal calcium absorbtion, bone calcium metabolism and probably, muscle activity.
Relevant Products
-
Decanoyl/octanoyl-glycerides
CAS:65381-09-1
-
Spinosad
CAS:131929-60-7
-
Regadenoson
CAS:313348-27-5