497871-47-3
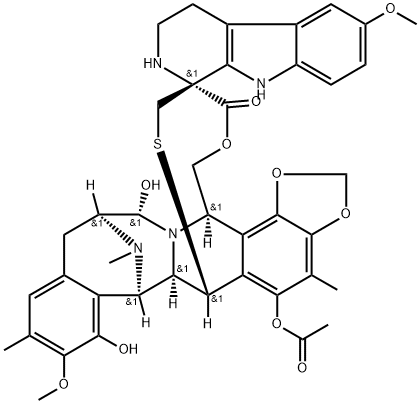
- Product Name:Lurbinectedin
- Molecular Formula:C41H44N4O10S
- Purity:99%
- Molecular Weight:784.88
Product Details:
CasNo: 497871-47-3
Molecular Formula: C41H44N4O10S
Appearance: White to Light Yellow Solid
Delivery Time: 2 weeks after order
Throughput: 100KG/Month
Purity: 99%
Synonyms: PM 01183; PM01183; PM-01183; Lurbinectidine; Spiro[6,16-(epithiopropanoxymethano)-7,13-imino-12H-1,3-dioxolo[7,8]isoquino[3,2-b][3]benzazocine-20,1'-[1H]pyrido[3,4-b]indol]-19-one, 5-(acetyloxy)-2',3',4',6,6a,7,9',13,14,16-decahydro-8,14-dihydroxy-6',9-dimethoxy-4,10,23-trimethyl-, (1'R,6R,6aR,7R,13S,14S,16R)- (9CI)
Density: 1.55±0.1 g/cm3
Pka: 9.79±0.40
Solubility: Soluble in DMSO
Description:
Lurbinectedin is a synthetic tetrahydropyrrolo [4, 3, 2-de]quinolin-8(1H)-one alkaloid analogue with potential antineoplastic activity. Lurbinectedin covalently binds to residues lying in the minor groove of DNA, which may result in delayed progression through S phase, cell cycle arrest in the G2/M phase and cell death.
Uses:
Lurbinectedin is being developed for the treatment of various cancers. It has been granted approval for treatment of metastatic small cell lung cancer.
Application:
Lurbinectedin is indicated for the treatment of adult patients with metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy.
Biological Activity:
Lurbinectedin a DNA minor groove covalent binder with potent in vitro and in vivo anti-tumour activity. Lurbinectedin inhibits RMG1 and RMG2 cell growth with IC50 of 1.25 nM and 1.16 nM, respectively.
Mechanism of action:
Lurbinectedin is a next-generation DNA minor groove binder that exerts potent antitumor activity in a low nanomolar range. Furthermore, it inhibits RNA-polymerase-II activity and promotes its specific degradation by the ubiquitin/proteasome machinery. In several human cancer cell lines, lurbinectedin blocks the transcription process through binding to CG-rich sequences near the promoters of protein-coding genes. This drug triggers both the degradation of phosphorylated RNA polymerase II (Pol II) on the DNA template and the generation of SSBs and DSBs that drive tumor cells to apoptosis. By attenuating the activity of the nucleotide excision repair (NER) mechanism, lurbinectedin was able to overcome cisplatin resistance in NER hyperactive cell lines. Lurbinectedin was also shown to inactivate Ewing Sarcoma Oncoprotein (EWS-FLI1) by nuclear redistribution leading to promotor inactivity and decreased mRNA and protein levels.
Relevant Products
-
Sparsentan
CAS:254740-64-2
-
Spinosad
CAS:131929-60-7