536-43-6
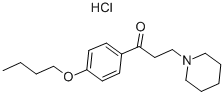
- Product Name:Dyclonine hydrochloride
- Molecular Formula:C18H28ClNO2
- Purity:99%
- Molecular Weight:325.87
Product Details:
CasNo: 536-43-6
Molecular Formula: C18H28ClNO2
Appearance: White Solid
Delivery Time: 2 weeks after order
Throughput: 100KG/Month
Purity: 99%
Synonyms: DYCLONINE HCL; 1-(4-BUTOXYPHENYL)-3-(1-PIPERIDINYL)-1-PROPANONE HCL; 1-(4-BUTOXYPHENYL)-3-(1-PIPERIDINYL)-1-PROPANONE HYDROCHLORIDE; 1-(4-butoxyphenyl)-3-(1-piperidinyl)-1-propanon hydrochloride; dyclonine hydrochlorideinoline-3-carboxylic acid; 1-(4-butoxyphenyl)-3-(1-piperidinyl)-1-propanone chloride
Solubility: Soluble in DMSO (20 mg/ml); Water (25 mg/ml)
Water Solubility: Partly soluble in water. Soluble in chloroform, ethanol, acetone and methanol.
Stability: Stable for 2 years from date of purchase as supplied. Solutions in DMSO or distilled water may be stored at -20°C for up to 2 months.
Description: Dyclonine hydrochloride is the hydrochloride salt of Dyclonine. Dyclonine belongs to a kind of oral anaesthetic. It is contained in the “Sucrets” as well as some kinds of Cepacol sore throat spray as an active ingredient. Dyclonine takes effect through reversibly binding to the activated sodium channels located on the neuronal membrane. This decreases the permeability of sodium across the neuronal membrane, further increasing the threshold for excitation. Overall, Dyclonine stabilizes the membrane and inhibits depolarization, resulting in the failure of a propagated action potential and further suppressing the subsequent conduction. This causes a transient and reversible anaesthetic effect in a local area of the body.
Chemical Properties: Dyclonine hydrochloride is a white crystalline powder possessing topical anesthetic properties characterized by rapid onset of effect, lack of systemic toxicity and a low index of sensitization.
Uses: Dyclonine hydrochloride is used for the temporary relief of the following occasional mouth and throat symptoms: pain, minor irritation, sore mouth and sore throat. It is a sodium channel blocker and a HSP90 chaperone protein inhibitor. It shows local anesthetic effects in vivo and is orally active.
Relevant Products
-
Milvexian
CAS:1802425-99-5
-
Isosorbide dinitrate
CAS:87-33-2
-
Cholic acid
CAS:81-25-4