521284-22-0
- Product Name:(alphaR)-alpha-[[[2-(4-Aminophenyl)ethyl]amino]methyl]benzenemethanol hydrochloride
- Molecular Formula:C16H20N2O.ClH
- Purity:99%
- Molecular Weight:292.809
Product Details:
CasNo: 521284-22-0
Molecular Formula: C16H20N2O.ClH
To see more products, please check our platform website--www.huarongpharm.com
Factory Sells 521284-22-0 Safe Transportation, Quality Manufacturer Supply (alphaR)-alpha-[[[2-(4-Aminophenyl)ethyl]amino]methyl]benzenemethanol hydrochloride
- Molecular Formula:C16H20N2O.ClH
- Molecular Weight:292.809
- Vapor Pressure:0.015Pa at 25℃
- PSA:58.28000
- Density:1145 at 20℃
- LogP:3.90860
(alphaR)-alpha-[[[2-(4-Aminophenyl)ethyl]amino]methyl]benzenemethanol hydrochloride(Cas 521284-22-0) Usage
| Description | (alphaR)-alpha-[[[2-(4-Aminophenyl)ethyl]amino]methyl]benzenemethanol hydrochloride is a complex organic molecule with an alpha stereoisomeric configuration at the alpha carbon. It contains an aminophenylethyl group linked to a benzenemethanol moiety, indicating its potential for biological activity, particularly in pharmaceutical research for drug development and receptor interactions. Hangzhou Huarong Pharm Co., Ltd. established since 2009 , has been always focusing on supplying products and services to our clients in the field of small molecule drug. Huarong Pharm has built platforms for the research, development and manufacturing of Building Blocks, Reference Compounds & Impurities, Natural products, APIs & Intermediates, Antibody-drug Conjugates (ADCs) and others. To maintain a high innovation efficiency, the company has continuously increased the investment on R&D facilities and state-of-the-art equipment in the past several years, including the establishment of kilogram GMP conditions plants and R&D centers. |
|
Uses |
(alphaR)-alpha-[[[2-(4-Aminophenyl)ethyl]amino]methyl]benzenemethanol hydrochloride is classified as an organic compound used in medicinal chemistry and chemical research. It likely falls under chemicals used in pharmaceutical development and neuroactive compounds due to its structural features. Utilized as a reference standard in the development and evaluation of drugs. It aids in ensuring quality control and consistency in pharmaceutical products. Potential applications in central nervous system or cardiovascular system drugs due to its structural resemblance to compounds that interact with neurotransmitter systems or adrenergic receptors. |
| Solubility | The hydrochloride salt form likely increases its solubility in water, a common trait for drug candidates and biochemical research compounds. |
| Stability | Requires storage in a dark place under an inert atmosphere to prevent degradation, indicating sensitivity to light or air. |
| Optical activity | (alphaR)-alpha-[[[2-(4-Aminophenyl)ethyl]amino]methyl]benzenemethanol hydrochloride has a stereoisomeric form with the R configuration at the alpha carbon, affecting its interaction with biological targets. |
|
Synthesis |
(alphaR)-alpha-[[[2-(4-Aminophenyl)ethyl]amino]methyl]benzenemethanol hydrochloride is synthesized from (R)-2-[2’ -(4-nitrophenyl)ethyl]amino]-1-phenylethanol monohydrochloride, involving hydrogenation with Raney Nickel, followed by a series of solvent treatments and crystallization steps to yield the hydrochloride salt form with a high yield and purity (86.1% yield, 99.14% purity). |
InChI:InChI=1S/C16H20N2O.ClH/c17-15-8-6-13(7-9-15)10-11-18-12-16(19)14-4-2-1-3-5-14;/h1-9,16,18-19H,10-12,17H2;1H/t16-;/m0./s1
521284-22-0 Relevant articles
A new process for the preparation of (R)-2-((4-Aminophenethyl)amino)-1-phenylethanol
-
Paragraph 0064-0068, (2021/07/01)
The present invention relates to a proce...
Systematic Investigation into the Formation of Significant Amounts of Unknown Impurity during Scale-up of NaBH4-I2 Mediated Reduction of Nitro-Amide Intermediate of Mirabegron No.
Bangal, Mukund N.,Deshmukh, Dattatray G.,Kalawade, Kaustubh A.,Mathad, Vijayavitthal T.
, p. 286 - 293 (2020/03/10)
After successful development of a manufa...
A PROCESS FOR PREPARATION OF MIRABEGRON AND ALPHA CRYSTALLINE FORM THEREOF
-
Page/Page column 33; 34; 35; 36, (2015/04/15)
An improved process for the preparation ...
AN IMPROVED PROCESS FOR THE PREPARATION OF 2-(2-AMINOTHIAZOL-4-YL)-N-[4-(2-[[(2R)-2-HYDROXY-2- PHENYLETHYL]AMINO]-ETHYL)PHENYL]ACETAMIDE
-
Page/Page column 19; 20, (2015/11/17)
The present invention relates to an impr...
521284-22-0 Process route
-

- 29968-78-3
2-(4-nitrophenyl)ethylamine monohydrochloride

-
![(R)-2-[ [2-(4-aminophenyl)ethyl]-amino]-1-phenylethanol monohydrochloride](/upload/2024/1/81b3b18d-4353-4956-91dc-c2f95157f0d0.png)
- 521284-22-0
(R)-2-[ [2-(4-aminophenyl)ethyl]-amino]-1-phenylethanol monohydrochloride
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1.1: Trimethyl borate; N-ethyl-N,N-diisopropylamine / acetonitrile / 8 h / Reflux
2.1: sodium tetrahydroborate / tetrahydrofuran / 25 °C
2.2: 11 h / 0 - 20 °C / Reflux
2.3: 20 - 25 °C
3.1: hydrogen / methanol / 2 h / 20 °C
With sodium tetrahydroborate; Trimethyl borate; hydrogen; N-ethyl-N,N-diisopropylamine; In tetrahydrofuran; methanol; acetonitrile;
|
-

- 611-71-2
(R)-Mandelic Acid

-
![(R)-2-[ [2-(4-aminophenyl)ethyl]-amino]-1-phenylethanol monohydrochloride](/upload/2024/1/81b3b18d-4353-4956-91dc-c2f95157f0d0.png)
- 521284-22-0
(R)-2-[ [2-(4-aminophenyl)ethyl]-amino]-1-phenylethanol monohydrochloride
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1.1: Trimethyl borate; N-ethyl-N,N-diisopropylamine / acetonitrile / 8 h / Reflux
2.1: sodium tetrahydroborate / tetrahydrofuran / 25 °C
2.2: 11 h / 0 - 20 °C / Reflux
2.3: 20 - 25 °C
3.1: hydrogen / methanol / 2 h / 20 °C
With sodium tetrahydroborate; Trimethyl borate; hydrogen; N-ethyl-N,N-diisopropylamine; In tetrahydrofuran; methanol; acetonitrile;
|
521284-22-0 Upstream products
-
521284-21-9

(R)-2-[[2′-(4-nitrophenyl)ethyl]amino]-1-phenylethanol hydrochloride
-
29968-78-3

2-(4-nitrophenyl)ethylamine monohydrochloride
-
611-71-2

(R)-Mandelic Acid
-
521284-19-5
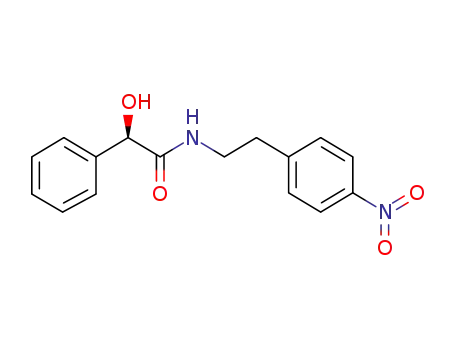
(2R)-2-hydroxy-N-[2-(4-nitrophenyl)ethyl]-2-phenylethanamide
521284-22-0 Downstream products
-
223673-61-8

(R)-2-(2-aminothiazol-4-yl)-4'-[2-[(2-hydroxy-2-phenylethyl)amino]ethyl]acetanilide
Relevant Products
-
Moxifloxacine Hcl
CAS:186826-86-8
-
Topiroxostat
CAS:577778-58-6
-
Terbinafine Hcl
CAS:78628-80-5