
128-08-5
- Product Name:N-Bromosuccinimide
- Molecular Formula:C4H4BrNO2
- Purity:99%
- Molecular Weight:177.985
Product Details:
CasNo: 128-08-5
Molecular Formula: C4H4BrNO2
Appearance: white to off-white powder
To see more products, please check our platform website--www.huarongpharm.com
Buy Quality N-Bromosuccinimide,Sale 128-08-5 On Stock
- Molecular Formula:C4H4BrNO2
- Molecular Weight:177.985
- Appearance/Colour:white to off-white powder
- Vapor Pressure:0.107mmHg at 25°C
- Melting Point:173- 176°C
- Refractive Index:1.606
- Boiling Point:221.4 °C at 760 mmHg
- PKA:-2.78±0.20(Predicted)
- Flash Point:87.7 °C
- PSA:37.38000
- Density:2.042 g/cm3
- LogP:0.38320
N-Bromosuccinimide(Cas 128-08-5) Usage
|
Description |
N-Bromosuccinimide (NBS) is a five-membered cyclic dicarboximide compound containing a bromine atom attached to the nitrogen atom. It is commonly used to generate bromine radicals (Br•) and is a versatile reagent for bromination in both organic synthesis and oxidation processes
|
|
Chemical Properties |
white to light yellow crystalline powder Soluble in organic solvents such as acetone and dimethylformamide. |
|
Uses |
N-Bromosuccinimide (NBS) is categorized as a brominating agent and an oxidizing reagent in organic chemistry. It is primarily used in radical substitution and electrophilic addition reactions. NBS is widely used for selective bromination at the allylic or benzylic positions of organic molecules. For example, NBS can brominate anthracene at the 9th position. NBS acts as an oxidizing agent in various oxidation reactions, including the oxidation of metal complexes such as Co(II) to Co(III). Huarong Pharm adheres to our vision, our mission, and our value, keeping abreast of the current trend and state-of-the-art science and technologies of the global biopharmaceutical industry to serve our clients to the utmost satisfaction. Our existing advantages have led to our in-depth services for the R&D of small molecule drug discovery. |
InChI:InChI=1/C4H4BrNO2/c5-6-3(7)1-2-4(6)8/h1-2H2
128-08-5 Relevant articles
Incompatibilities between N-Bromosuccinimide and Solvents
Sumio Shimizu*, Yoshiaki Imamura, and Tatsuo Ueki
, Org. Process Res. Dev. 2014, 18, 2, 354–358
Incompatibilities between N-bromosuccinimide (NBS) and solvents were examined by measuring the heat of reaction by an advanced reactive system screening tool (ARSST) and a reaction calorimeter (RC1e). The ARSST experiments showed that amides, THF, and toluene solutions require consideration of incompatibilities with NBS. Isothermal analyses by RC1e revealed accurate heats of incompatibilities and the autocatalytic behaviors of the incompatibilities.
Organic Synthesis Using Sodium Bromate. II. A Facile Synthesis of N-Bromo Imides and Amides Using Sodium Bromate and Hydrobromic Acid (or Sodium Bromide) in the Presence of Sulfuric Acid
Fujisaki, Shizuo,Hamura, Satoshi,Eguchi, Hisao,Nishida, Akiko
, p. 2426 - 2428 (1993)
The reaction of imides and amides in wat...
Evaluation of a Continuous-Flow Photo-Bromination Using N-Bromosuccinimide for Use in Chemical Manufacture
Matthew Waterford A B , Simon Saubern and Christian H. Hornung
Australian Journal of Chemistry 74(8) 569-573
A continuous-flow photo-bromination reaction on benzyl and phenyl groups was conducted using N-bromosuccinimide as the bromine source inside a preparatory-scale glass plate reactor. This flow reactor system was capable of independently controlling light intensity, wavelength, and reaction temperature, hence exerting an exceptional level of control over the reaction.
128-08-5 Upstream products
-
123-56-8
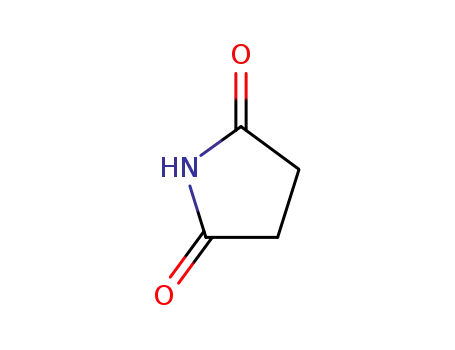
Succinimide
-
79-15-2

N-bromoacetamide
-
128-09-6

N-chloro-succinimide
-
82815-39-2

benzotelluracyclopentane bromide succinimide
128-08-5 Downstream products
-
528-58-5
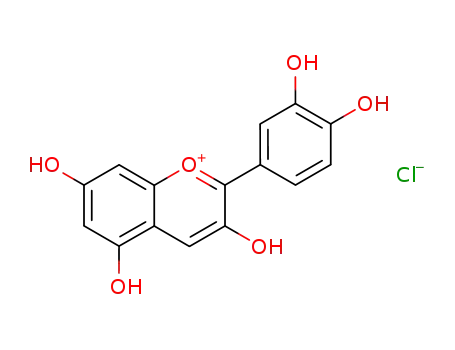
cyanidin chloride
-
617-35-6
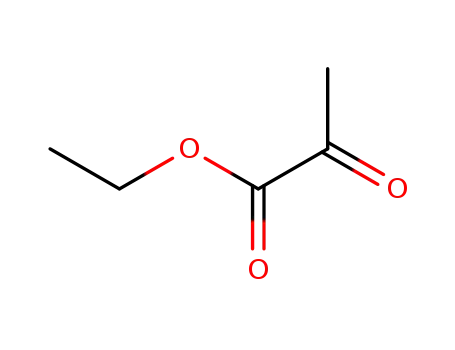
2-oxo-propionic acid ethyl ester
-
759-05-7

3-methyl-2-ketobutanoic acid
-
78-84-2

isobutyraldehyde
Relevant Products
-
Aprocitentan
CAS:1103522-45-7
-
Sotorasib
CAS:2296729-00-3








