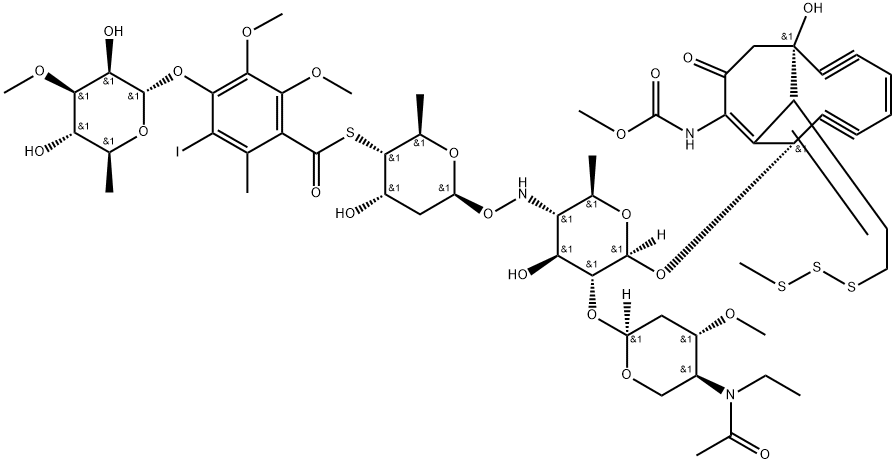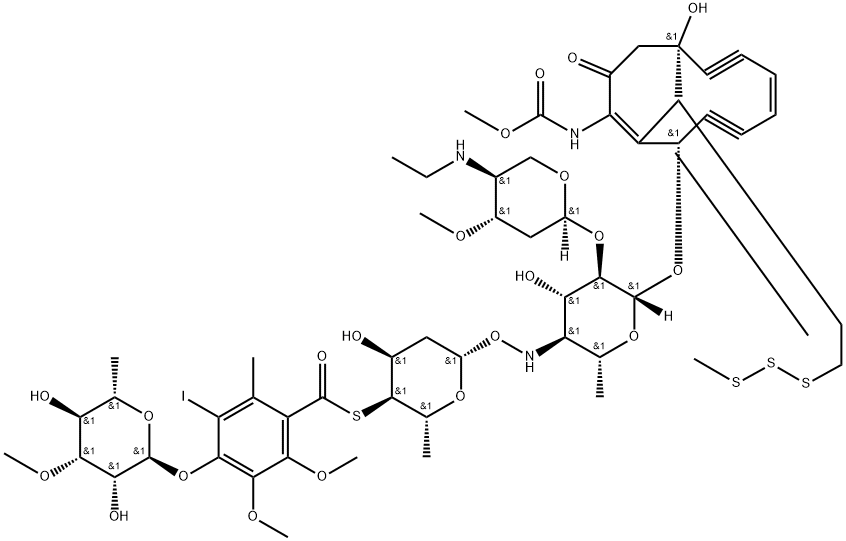
108212-75-5
- Product Name:Calicheamicin
- Molecular Formula:C55H74IN3O21S4
- Purity:99%
- Molecular Weight:1368.34
Product Details:
CasNo: 108212-75-5
Molecular Formula: C55H74IN3O21S4
Appearance: White to yellow solid
Delivery Time: 2 weeks after order
Throughput: 100KG/Month
Purity: 99%
Synonyms: calicheamicin gamma(1)I; Benzenecarbothioic acid, 4-((6-deoxy-3-o-methyl-alpha-L-mannopyranosyl)oxy)-3-iodo-5,6-dimethoxy-2-methyl-, 4''-ester with methyl (8-((4,6-dideoxy-2-o-(2,4-dideoxy-4-(ethylamino)-3-o-methyl-alpha-L-threo-pentapyranosyl)-4-(((2,6-dideoxy-4-thio-beta-D-ribo-hexopyranosyl)oxy)amino)-beta-D-glucopyranosyl)oxy)-1-hydroxy-13-(2-(methyltrithio)ethylidene)-11-oxobicyclo(7.3.1)trideca-4,9-diene-2,6-diyn-10-yl)carbamate, (1R-(1R*,4Z,8S*,13E))-; Calichemicin gamma1; Carbamic acid, ((1R,4Z,8S,13E)-8-((4,6-dideoxy-4-(((2,6-dideoxy-4-S-(4-((6-deoxy-3-o-methyl-alpha-L-mannopyranopyranosyl)oxy)-3-iodo-5,6-dimethoxy-2-methylbenzoyl)-4-thio-beta-D-ribo-hexopyranosyl)oxy)amino)-2-o-(2,4-dideoxy-4-(ethylamino)-3-o-methyl-alpha-L-threo-pentopyranosyl)-beta-D-glucopyranosyl)oxy)-1-hydroxy-13-(2-(methyltrithio)ethylidene)-11-oxobicyclo(7.3.1)trideca-4,9-diene-2,6-diyn-10-yl)-, methyl ester;Calicheamicin γ1; LL-E 33288 gamma1-I; calicheamicin gamma; Calicheamicin γ1
Alpha: D26-124° (c = 0.98%, EtOH)
Density: 1.57±0.1 g/cm3
Solubility: DMSO : ≥ 100 mg/mL (73.08 mM);Water : < 0.1 mg/mL (insoluble)
Pka: 7.13±0.60
Description:
Calicheamicins are a series of enediyne antitumor antibiotics originally isolated from the bacterium Micromonospora echinospora. They exert high cytotoxicity and have been applied in tumor therapies for over a decade-- a CD33 antigen-targeted immuno-conjugate: N-acetyl dimethyl hydrazide calicheamicin, was developed as a targeted therapy for non-solid tumor cancer acute myeloid leukemia (AML).
Uses:
Calicheamicins are a class of enediyne antitumor antibiotics derived from the bacterium Micromonospora echinospora, The main component is calicheamicin γ1. It has strong anti-Gram-positive bacteria activity and anti-tumor effect.
Structure:
Calicheamicin represents one of the most structurally complex natural products developed into an anticancer agent. After binding to the minor groove of DNA with some sequence specificity, calicheamicin is reduced by cellular thiols.
Relevant Products
-
N-Acetyl-Calicheamicin
CAS:108212-76-6
-
Ansamitocin P-3
CAS:66584-72-3
-
Mertansine
CAS:139504-50-0