
122111-03-9
- Product Name:Gemcitabine HCl
- Molecular Formula:C9H11F2N3O4.HCl
- Purity:99%
- Molecular Weight:299.662
Product Details:
CasNo: 122111-03-9
Molecular Formula: C9H11F2N3O4.HCl
Appearance: White crystalline granular, odorless
Chinese Factory Supply High Purity Gemcitabine HCl 122111-03-9 Lowest Price
- Molecular Formula:C9H11F2N3O4.HCl
- Molecular Weight:299.662
- Appearance/Colour:White crystalline granular, odorless
- Vapor Pressure:2.41E-11mmHg at 25°C
- Melting Point:>250 °C dec
- Boiling Point:482.7 °C at 760 mmHg
- Flash Point:245.7 °C
- PSA:110.60000
- Density:==
- LogP:0.09460
Gemcitabine HCl (Cas 122111-03-9) Usage
| Category | Gemcitabine hydrochloride (GEM HCl) is classified as a chemotherapy drug under the category of antimetabolites. |
| Definition | Gemcitabine hydrochloride is a difluoro analog of deoxycytidine. It is a nucleoside analog that interferes with DNA synthesis, inhibiting the growth and proliferation of cancer cells. By inhibiting ribonucleotide reductase, it reduces the availability of cellular nucleotides necessary for DNA replication. Gemcitabine is widely used as part of chemotherapy regimens for treating cancers such as non-small cell lung cancer, pancreatic cancer, and breast cancer. |
| Properties | Solubility: Soluble in water Practically insoluble in ethanol and other organic solvents Pharmacokinetics: Half-life: Gemcitabine has a short half-life and is rapidly deactivated by cytidine deaminase in the plasma and liver. Metabolism: Undergoes intracellular phosphorylation to active forms (gemcitabine diphosphate and triphosphate), inhibiting DNA replication. Excretion: Primarily excreted through the kidneys. |
| Uses |
Cancer Treatment: Hangzhou Huarong Pharm Co., Ltd. established since 2009 , has been always focusing on supplying products and services to our clients in the field of small molecule drug. Huarong Pharm has successfully delivered innovative R&D products and services to more than 3,000 partners across the world. Our goal is to become a world-class leading company to support life science innovation and manufacturing. |
| Side Effects and Toxicity | Myelosuppression: Gemcitabine can cause significant bone marrow suppression, leading to anemia, leukopenia, and thrombocytopenia. Liver Function: Approximately 66% of patients experience liver enzyme abnormalities, which are usually mild. Renal Toxicity: Some patients may experience proteinuria, hematuria, and, in rare cases, renal failure. Skin Reactions: Approximately 25% of patients develop skin rashes; itching occurs in around 10% of patients. Pulmonary Effects: Less than 1% of patients may experience bronchospasms. Other Side Effects: Hair thinning (but not complete loss) Nausea and vomiting in approximately 33% of patients Fluid retention leading to swelling in the extremities and face Serious side effects: Vision changes, mood alterations, seizures |
| Biological Activity | Gemcitabine is metabolized into active forms (gemcitabine diphosphate and triphosphate), which inhibit ribonucleotide reductase, depleting cellular nucleotide levels. The triphosphate form is also incorporated into the DNA of cancer cells, causing strand termination, which prevents DNA replication and promotes apoptosis. This mechanism underlies gemcitabine's broad antitumor activity both in vitro and in vivo. |
InChI:InChI=1/C9H11F2N3O4.ClH/c10-9(11)6(16)4(3-15)18-7(9)14-2-1-5(12)13-8(14)17;/h1-2,4,6-7,15-16H,3H2,(H2,12,13,17);1H
122111-03-9 Relevant articles
Antitumor activity of gemcitabine hydrochloride loaded lipid polymer hybrid nanoparticles (LPHNs): In vitro and in vivo
Tahir Emre Yalcin , Sibel Ilbasmis-Tamer , Sevgi Takka
, International Journal of Pharmaceutics Volume 580 , 30 April 2020, 119246
This study offers GEM loaded LPHNs to prolong its half-life and reduce its side effects and compares the in vivo antitumoral efficacy of our formulation with the commercial product Gemko®. In vivo antitumor effects were observed on nitrosomethylurea-induced mammary tumors in female Sprague-Dawley rats. Cytotoxicities of the LPHNs were evaluated on MCF-7 and MDA-MB-231 cells by MTT assays. The comparative pharmacokinetics of Gemko® and GEM loaded LPHNs formulation was also evaluated in rats.
Long circulating polymeric nanoparticles of gemcitabine HCl using PLGA-PEG-PPG-PEG block co-polymer
Ketaki Kale,Aditi Fulfager,Kapil Juvale &Khushwant S. Yadav
, International Journal of Polymeric Materials and Polymeric Biomaterials Volume 73, 2024 - Issue 3
In this study, Gemcitabine (GEM) HCl is loaded in long-circulating polymeric nanoparticles (NPs) using PLGA- PEG-PPG-PEG in combination with succinic anhydride (SA) to overcome issues of rapid metabolism. The optimized batch had size of 230.9 nm, zeta potential of −15.4 mV, and a high entrapment efficiency (EE) of 69.69%.
Degradation Chemistry of Gemcitabine Hydrochloride, a New Antitumor Agent
Sally L. Anliker, Michael S. McClure, Thomas C. Britton, Erwin A. Stephan, Steven R. Maple, Gary G. Cooke
Journal of Pharmaceutical Sciences Volume 83, Issue 5, May 1994, Pages 716-719
Approximately 72% of the initial gemcitabine remains after 4 weeks under the basic conditions used. Uridine hydrolysis products are also formed under these conditions. The anomerization reaction, which is unusual under basic conditions, has been confirmed by characterization of the chromatographically isolated α‐anomer by NMR and mass spectrometry.
122111-03-9 Process route
-

- 1445381-44-1
(2R,3R,5R)-5-(4-benzamido-2-oxopyrimidin-1(2H)-yl)-2-((benzoyloxy)methyl)-4,4-difluorotetrahydrofuran-3-yl benzoate

-
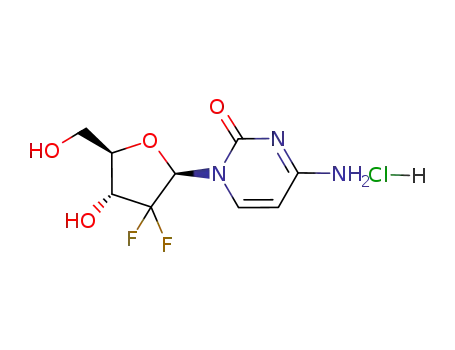
- 122111-03-9
gemcitabine hydrochloride
| Conditions | Yield |
|---|---|
|
(2R,3R,5R)-5-(4-benzamido-2-oxopyrimidin-1(2H)-yl)-2-((benzoyloxy)methyl)-4,4-difluorotetrahydrofuran-3-yl benzoate; With ammonia; In methanol; at 20 ℃; for 6h; Inert atmosphere;
With hydrogenchloride; In water; isopropyl alcohol; at 20 ℃; pH=2; Heating;
|
90% |
|
With sodium methylate; In methanol; at 20 ℃; for 4h; Reagent/catalyst;
|
90% |
|
(2R,3R,5R)-5-(4-benzamido-2-oxopyrimidin-1(2H)-yl)-2-((benzoyloxy)methyl)-4,4-difluorotetrahydrofuran-3-yl benzoate; With ammonium hydroxide; In methanol; at 20 ℃; for 16h;
With hydrogenchloride; In water; isopropyl alcohol; at 20 ℃;
|
78% |
-
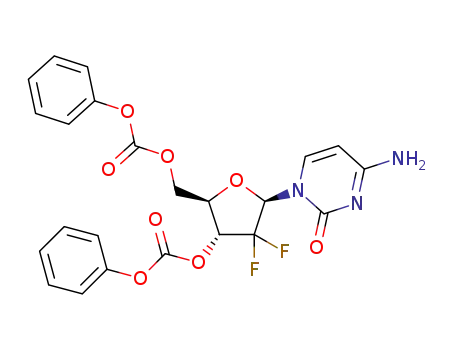
-
3',5'-di-O-benzoyl-β-2'-deoxy-2',2'-difluorocytidine

-

- 122111-03-9
gemcitabine hydrochloride
| Conditions | Yield |
|---|---|
|
3',5'-di-O-benzoyl-β-2'-deoxy-2',2'-difluorocytidine; With ammonium hydroxide; water; In methanol; for 6h; Reflux;
With hydrogenchloride; In methanol; water; at 20 ℃; for 12h; pH=3;
|
85% |
122111-03-9 Upstream products
-
250698-52-3
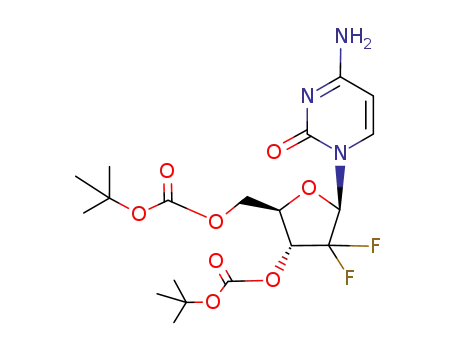
3′,5′-O-bis(tert-butoxycarbonyl)-gemcitabine
-
951314-37-7

α/β-1-(2-oxo-4-amino-1H-pyrimidin-1-yl)-2-desoxy-2,2'-difluororibose hydrochloride
-
103882-84-4

2’-deoxy-2’,2’-difluoro-cytidine
-
95058-81-4

gemcitabine
122111-03-9 Downstream products
-
114248-23-6

Deoxydifluorouridine
-
95058-85-8
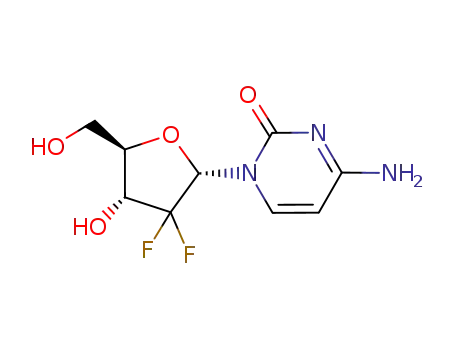
α-1-(2-oxo-4-amino-1,2-dihydropyrimidin-1-yl)-2-deoxy-2,2-difluororibose
-
250698-60-3
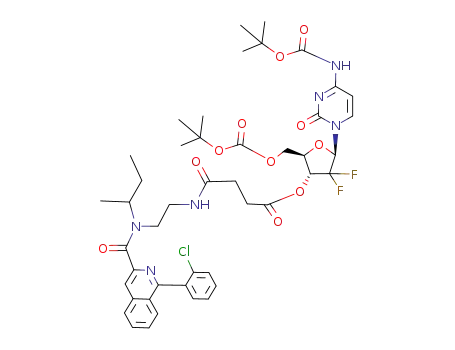
4-N-5'-O-bis(tert-butoxycarbonyl)-3'-O-[2-[2-[N-(1-methylpropyl),N-[1-(2-chlorophenyl)-isoquinoline-3-carbonyl]amino]ethylaminocarbonyl]ethylcarbonyl]gemcitabine
-
250698-59-0
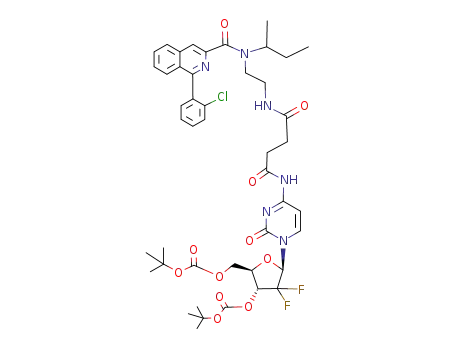
3'-O-5'-O-bis(tert-butoxycarbonyl)-4-N-[2-[2-[N-(1-methylpropyl),N-[1-(2-chlorophenyl)-isoquinoline-3-carbonyl]amino]ethylaminocarbonyl]ethylcarbonyl]gemcitabine
Relevant Products
-
Moxifloxacine Hcl
CAS:186826-86-8
-
Pazopanib Hcl
CAS:635702-64-6
-
Valbenazine base
CAS:1025504-45-3








